Treatments for CSID
In patients with Congenital Sucrase-Isomaltase Deficiency (CSID) and gastrointestinal symptoms that warrant treatment, three major treatment options exist:
- Severe diet restriction
- Sucraid® (sacrosidase) Oral Solution
- Sucraid® with moderate diet restriction
Before Sucraid® was available, severe restriction of dietary carbohydrate intake was the only treatment option for patients with CSID.
Given the sucrose content of Western diets and the difficulties of dietary adherence generally, patient compliance with a sucrose-free diet is challenging and often accompanied by chronic gastrointestinal symptoms and decreased body weight for a given height and age.1-3 In an observational study, only 50% of children with CSID complied with dietary reductions of sucrose and starch, and 60% to 75% still experienced frequent diarrhea and abdominal pain, presumably due to a lack of adherence to the sucrose elimination diet.4
Many Foods Contain Sucrose
The average American adult consumes about 130 pounds of sugar per year, about 70 pounds of which is sucrose.5 Sucrose can be found in a surprising number of foods. Sucrose is the naturally-occurring sugar in many fruits, juices, and some vegetables, and is the sugar often added in baked goods and other processed foods. Based on a consumption of 2,000 calories per day, sucrose makes up 10% of the total American diet.5
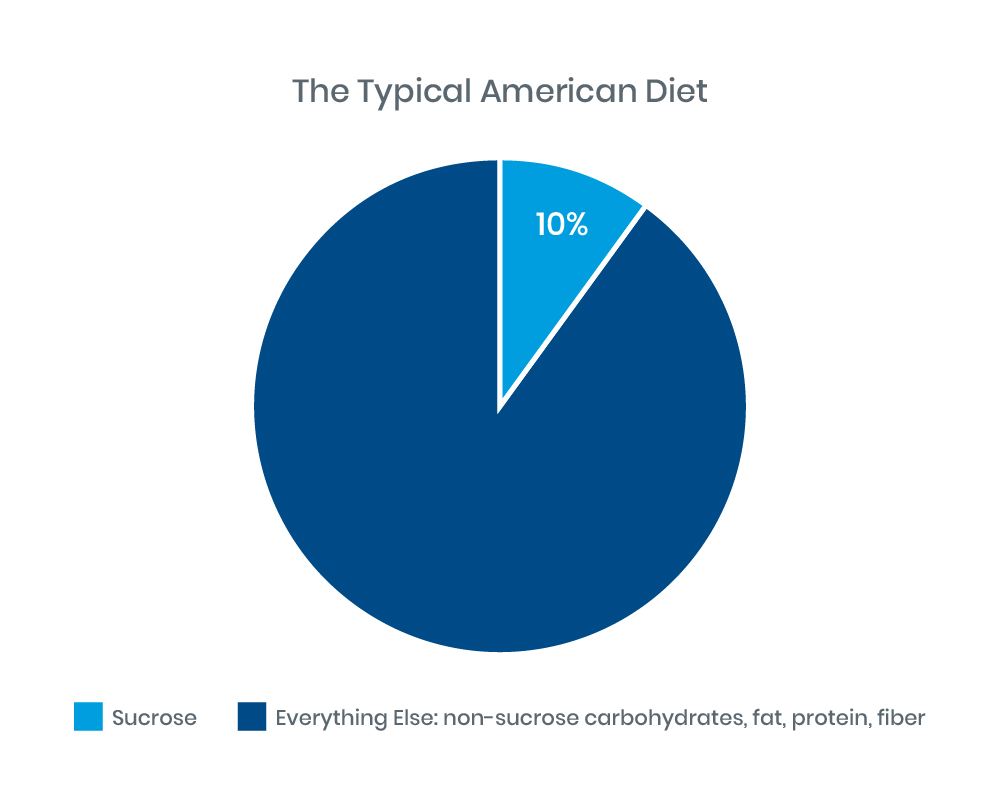 |
Figure 1: Adapted from Treem 2012: J Pediatr Gastroenterol Nutr.4
Given the amount of sucrose Americans eat every year, it is no surprise that maintaining a low sucrose diet can be challenging. Patients with CSID must avoid eating a surprising number of foods, all of which are high in starches and/or sucrose.
The following is a list of foods with high levels of starch (denoted with *) and/or sugar (more than 1 gram of sucrose per 100 grams of food):
- Fruit: apples, apricots, bananas,* cantaloupe, dates, grapefruit, guava, honeydew melon, mango, nectarine, oranges, passion fruit, peaches, persimmon, pineapple, plums, tangelos, tangerines, watermelon
- Vegetables: beets, carrots, cassava (yucca),* chickpeas,* coleslaw, corn, edamame,* green peas,* jicama,* kidney beans,* lima beans,* okra, onion, parsnips,* pumpkin, snow peas, split peas,* sweet pickles, sweet potatoes,* yams*
- Dairy: chocolate and flavored milks, milk shakes, sweetened condensed milk, malted milk, yogurt sweetened with sucrose or with fruits containing sucrose, processed cheese or cheese products with additives, fillers or preservatives that may contain sucrose or starch
- Protein: prepared, processed, breaded, or cured meats; for example, bacon, sausage, luncheon meat, pate, liverwurst, tofu
- Nuts and seeds: almonds, cashews,* chestnuts,* filberts/hazelnuts, macadamia nuts, peanuts,* pecans, pine nuts, pistachios, nut butters with added sucrose, sesame seeds
- Other starches: baked goods sweetened with sucrose; for example, breads, cereals, snacks, muffins, pancakes, pastries, waffles
- Fats: some mayonnaises and salad dressings
- Sweeteners and ingredients: table, brown, granulated, powdered or raw sugar, beet sugar, cane sugar/syrup/juice, coconut sugar, date sugar, maple syrup/sugar, molasses, syrup, jelly, jam, brown rice syrup, caramel, corn syrup/syrup solids, dextrins, glucose polymers, maltodextrin, modified tapioca starch
- Desserts: ice cream, popsicles, pudding, sherbet, sorbet, brownies, cakes, cookies, muffins, pastries, pies, whipped toppings, carob, caramel, chocolate, chocolate sauce/syrup
- Beverages: all sucrose-sweetened beverages, juices made from fruits containing sucrose (see above), energy drinks, sports drinks, fruit-flavored drinks, sucrose-sweetened soda or drink mixes, specialty/flavored coffees containing sucrose
- Condiments: BBQ sauce, ketchup, chutney, seasonings or sauces containing sucrose, salad dressings, sweet pickles
Enzyme Replacement Therapy
Enzyme replacement therapy with Sucraid® (sacrosidase) Oral Solution offers a pharmacologic alternative to sucrose-free restricted diets. Sucraid® is the only FDA-approved therapy for the treatment of sucrase deficiency, which is part of CSID. Two key clinical trials demonstrated the ability of sacrosidase to significantly reduce breath hydrogen, which is an indirect measure of sucrose digestion, which can correlate with GI symptoms in CSID patients when challenged with a normal sucrose-containing diet.6,7 In the clinical trial that quantified treatment responses, 81% of 26 children with CSID aged 5 months to 11 years became asymptomatic with sacrosidase replacement of sucrase; asymptomatic was defined as having no watery stools and no or mild GI symptoms on 7 of the 10 study treatment days.6 In both studies, study participants who received higher doses of sacrosidase had fewer stools, a greater number of formed or hard stools, as well as fewer symptoms of gas, abdominal cramping, and bloating compared to those who received lower doses of sacrosidase.6,7 In a third clinical trial, conducted over a period of several years in 32 participants diagnosed with CSID, Sucraid® again demonstrated it is an effective option for the management of sucrase deficiency, reducing or eliminating the need for restrictions of dietary sucrose, as well as reducing or eliminating the symptoms of CSID in the majority of patients.8
It is important to note that CSID patients generally have a reduced capacity for the digestion of sugars from dietary starches due to reduced isomaltase and maltase enzyme activities. Sucraid® does not provide specific replacement therapy for deficient starch digestion.8 Therefore, restricting starch in the diet is usually necessary in patients with CSID. The need for dietary starch restriction in patients using Sucraid® should be evaluated on a patient-by-patient basis.
Proper dosing is essential for Sucraid® to be effective.
Recommended dosing for Sucraid® single-use containers:
The contents of the Sucraid® 2-ml single-use container should be diluted in 4 ounces of water, milk, or infant formula. Patients should consume:
- all of the mixed solution dose per meal or snack for patients over 33 lb (15 kg) in body weight
- half of the mixed solution dose per meal or snack for patients 33 lb (15 kg) and less
The appropriate dose of Sucraid® is to be taken orally with each meal or snack, diluted with 4 ounces (120 mL) of water, milk, or infant formula. The beverage or infant formula used to dilute Sucraid® should not be heated, nor should Sucraid® be added to hot beverages, as heat can decrease the potency of the enzyme. Sucraid® should not be reconstituted or consumed with fruit juices, as the acidity may impair enzyme activity. For patients 33 lb (15 kg) and less using the Sucraid® 2-ml single-use container, the second half of the mixed solution should be refrigerated and discarded if not used within 24 hours.
Single-use containers of Sucraid® can be removed from refrigeration and stored at 15°C to 25°C (59°F to 77°F) for up to 3 days (72 hours).
Practice Tip
The low-FODMAP diet is not recommended for patients with CSID. Although it is a standard diet for patients with irritable bowel syndrome (IBS), it will not address symptoms in patients with CSID since it contains many foods that are high in sucrose, including: banana, cantaloupe, grapefruit, honeydew melon, mandarin orange, orange, passion fruit, pineapple, tangelo, carrot, parsnip, potato, silver beet, sweet potato, taro, yam, gluten-free breads and cereals, 100% spelt bread, rice, oats, polenta, arrowroot, millet, psyllium, quinoa, sorghum, tapioca, lactose-free yogurt, gelato, sorbet, table sugar, golden syrup, maple syrup, and molasses.
- Treem WR. Congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 1995;21(1):1-14. doi:10.1097/00005176-199507000-00001
- Gudmand-Høyer E. Sucrose malabsorption in children: a report of thirty-one Greenlanders. J Pediatr Gastroenterol Nutr. 1985;4(6):873-877. doi:10.1097/00005176-198512000-00005
- Gudmand-Høyer E, Krasilnikoff PA, Skovbjerg H. Sucrose-isomaltose malabsorption. In: Draper H, ed. Adv Nutr Res. 1984;6:233-69. doi:10.1007/978-1-4613-2801-8_9
- Puntis JW, Zamvar V. Congenital sucrase-isomaltase deficiency: diagnostic challenges and response to enzyme replacement therapy. Arch Dis Child. 2015;100(9):869-871. doi:10.1136/archdischild-2015-308388
- US Department of Agriculture. U.S. consumption of caloric sweeteners. Last updated October 19, 2022. www.ers.usda.gov/webdocs/DataFiles/53304/Group%207%20Tables%20-%20US%20Caloric%20Sweetener%20Consumption.xlsx?v=359.3
- Treem WR, Ahsan N, Sullivan B, et al. Evaluation of liquid yeast-derived sucrase enzyme replacement in patients with sucrase-isomaltase deficiency. Gastroenterology. 1993;105(4):1061-1068. doi:10.1016/0016-5085(93)90950-h
- Treem WR, McAdams L, Stanford L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 1999;28(2):137-142. doi:10.1016/0016-5085(93)90950-h
- QOL Medical, LLC. Open-label, long-term study of sucrase enzyme therapy for congenital sucrase-isomaltase deficiency. Data on file, OMC-SUC-3. 1997. doi:10.1097/00005176-199902000-00008



